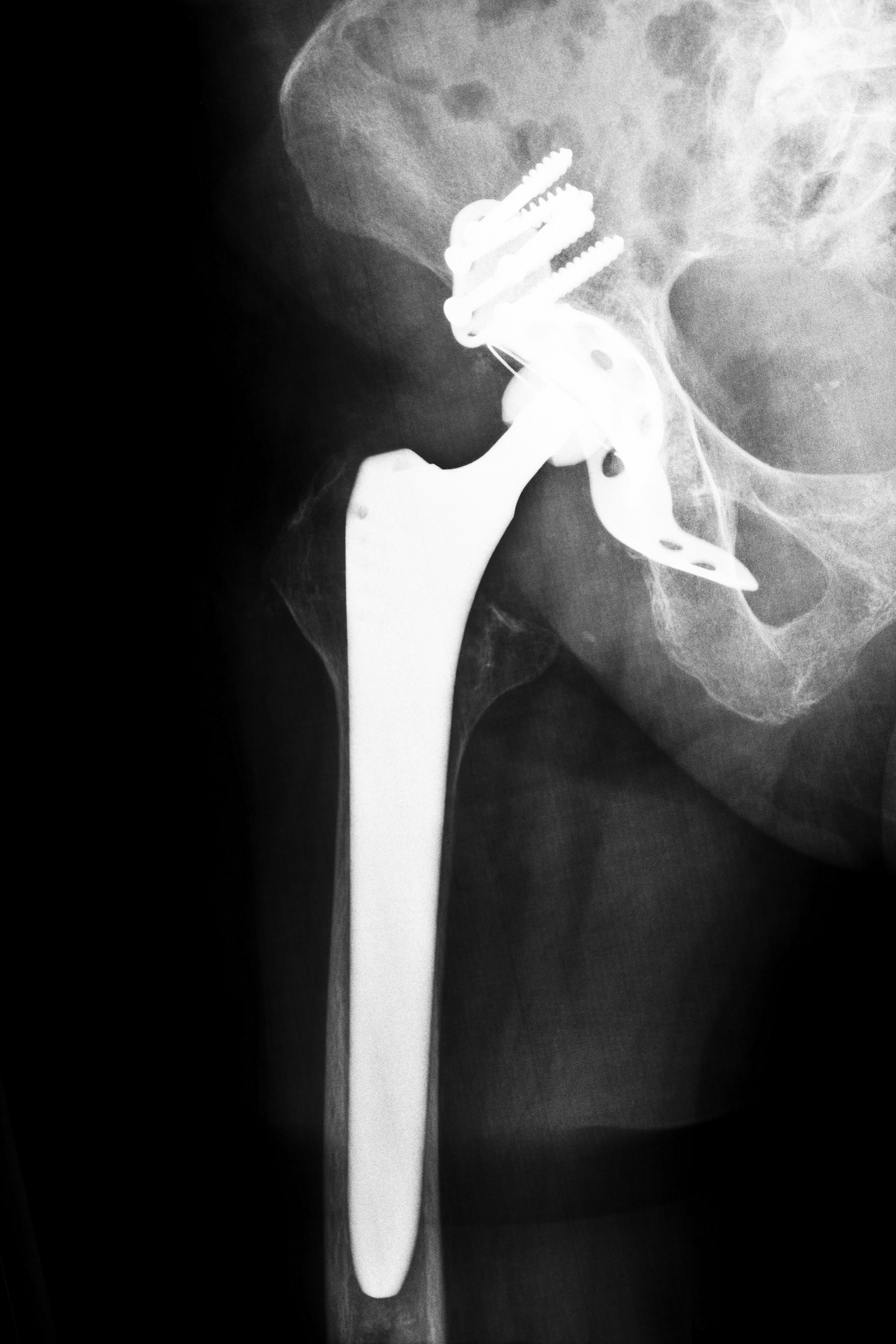
 An executive of DePuy Orthopaedics, a subsidiary of Johnson & Johnson, recently defended the company’s assessment of the potential safety hazards associated with its metal-on-metal Pinnacle hip replacement devices. On September 15, 2014, the representative faced a lawyer who alleged that the company had neglected to perform critical testing of the device’s safety and efficacy.
An executive of DePuy Orthopaedics, a subsidiary of Johnson & Johnson, recently defended the company’s assessment of the potential safety hazards associated with its metal-on-metal Pinnacle hip replacement devices. On September 15, 2014, the representative faced a lawyer who alleged that the company had neglected to perform critical testing of the device’s safety and efficacy.
An attorney for the plaintiff questioned DePuy’s vice president of clinical research during the first DePuy Pinnacle hip implant trial. According to court documents, there are more than 6,000 lawsuits pending involving Pinnacle hips, and the cases have been consolidated into a federal court in Dallas, Texas. The outcome of this first trial, also known as a bellwether case, could significantly impact the manufacturer’s decision to continue fighting lawsuits in court or to agree to settlements instead.
Plaintiffs accuse the company of neglecting to understand and warn doctors and patients about the risks and complications and instead marketed the DePuy Pinnacle hip implant as safe. During the trial, the plaintiff’s attorney asked the DePuy executive about the studies that DePuy had performed on the devices, attempting to show jurors that the manufacturer was negligent in gathering data on the implants before they received approval from the U.S. Food and Drug Administration in 2000.
According to the DePuy executive, no human study examined the risk of metallosis, or metal toxicity, in Pinnacle hip implants prior to 2001. The plaintiff’s attorney countered that suggested problems with metal-on-metal hip implants first came to light as early as 1974.
However, according to DePuy, the manufacturer adhered to industry standards and maintained that the company had conducted the necessary studies on the metal-ion issue to prove that its products were safe. Whether DePuy properly vetted the devices and the safety record of all-metal hip implants have been key themes during the bellwether trial so far, according to court documents.
The attorney for the plaintiffs claimed that the failure rates for the DePuy Pinnacle hip implant were unacceptably high – more than 14 percent over seven years. The plaintiff alleged received two Pinnacle hips in 2009, and claimed in her 2012 lawsuit that defects of the products caused the level of cobalt in her blood to soar to 85 times the normal level.
While the Pinnacle implant has not yet been recalled, DePuy stopped marketing metal liners for the implants in 2013. Last year, DePuy agreed to a $2.5 billion settlement to resolve more than 7,000 lawsuits involving its ASR all-metal hip systems, which were pulled from the market in 2010.
Have You Been Fitted with a DePuy Pinnacle Hip Implant? We Can Help
If you or someone you love received a DePuy Pinnacle hip implant and you believe that your complications were caused by the device, please contact Attorney Group today for a free, no-obligation case evaluation. We can help to answer your questions and put you in touch with a local affiliated lawyer who can help you to seek the compensation to which you may be entitled.