Kentucky Stryker Hip Recall Attorney Warns of Potential Stryker Hip Failure
If you have any Stryker hip product, you should contact a Kentucky Stryker hip recall attorney today. The Stryker ABG II Modular Neck Stem was recalled in July 2012 after the U.S. Food & Drug Administration (FDA) received many reports that this hip implant system was prone to corrosion that can cause serious side effects with surrounding tissue reactions. Some patients who received the ABG II Modular Neck hip system have reported complications of persistent pain and swelling at the site of the joint. Patients affected by the Stryker ABG II Modular Neck Hip Stem have started filing lawsuits seeking monetary compensation for injuries they have suffered after undergoing hip implant surgery. A Kentucky Stryker hip recall attorney affiliated with the Attorney Group for Kentucky can speak with recipients of hip implants to determine if they are eligible to file a Kentucky Stryker ABG II Recall lawsuit.
Symptoms and Complications with Stryker Hip Systems
According to Kentucky Stryker hip recall attorneys, the cause for concern associated with the Stryker ABG II modular neck stem is corrosion of the femoral parts. When metal components rub against other metal components, toxic metal particles are shed. They can seep into the bloodstream and surrounding tissue and bone causing metal poisoning, also called metallosis, tissue necrosis and other severe complications. Reports have shown that metal poisoning can also lead to damaged spleen, liver, kidneys and lymph nodes. Many complaints were reported to the U.S. Food & Drug Administration that recipients of Stryker hip systems were experiencing such complications as the following:
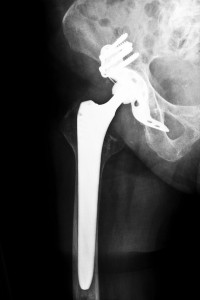
- Pain and swelling
- Surrounding tissue damage and tissue necrosis
- Metallosis, metal poisoning
- Osteolysis, bone resorption
- Fracture or loss of bone surrounding the joint
- Inflammation
- Failure of hip device
- Infections
- Implant loosening
- Pseudotumors
Patients who received a Stryker ABG II hip implant have been warned by Stryker to get imaging and blood tests even if they are not having any symptoms of side effects to evaluate the condition of their implant. After consulting with their doctor, many patients seek legal advice from a Kentucky Stryker hip recall attorney to make sure their legal rights are protected.
Kentucky Stryker ABG II Recall lawsuit
The Stryker ABG II Modular Neck Stem and the Stryker Rejuvenate are hip replacement systems that consist of a metal neck and a metal stem. These metal on metal hip replacement systems were put on the market in 2009 and an estimated 20,000 were sold worldwide before being recalled. If you are a recipient of a hip implant between 2009 and July 2012 and have experienced any complications, you could have received a Stryker ABG II Modular Neck Stem hip replacement system. Even if you are not experiencing any symptoms, you should speak with an experienced Stryker hip recall attorney affiliated with Attorney Group for Kentucky to discuss a possible Kentucky Stryker ABG II Recall lawsuit. Many patients who received metal on metal hip implants have been found to have high levels of metal ions in their blood even without experiencing any symptoms. Contact a Kentucky Stryker hip recall attorney at Attorney Group for Kentucky today so we can evaluate your case to determine the best course of action for you to take next to protect your rights.
For more information see our Stryker Hip Recall Attorney Information Page





